The Karmouty Lab
Uncovering the mechanisms behind Vascular Remodeling and Fibrosis
Pulmonary Diseases
According to the World Health Organization (WHO), pulmonary diseases are the fourth most common cause of death worldwide, and the third most common cause of death in the USA, Europe and Japan.

Idiopathic Pulmonary Fibrosis (IPF)
IPF is a lethal lung disease of
unknown etiology defined by chronic, progressive and irreversible interstitial fibrosis (scarring).
This scarring process slowly obliterates the alveoli of the lung, preventing patients to obtain oxygen from the air they breathe.
IPF has a high mortality rate of up to 80% underscoring the need to develop novel therapies to treat this fatal disease.
Our lab focuses in understanding new molecular pathways that are important in the development of pulmonary fibrosis.

Systemic Sclerosis Interstitial Lung Disease (SSc-ILD)
SSc has the highest
mortality of all connective
tissue diseases.
SSc is a devastating disease characterized by skin and internal organ fibrosis. ILD is the leading cause of death in SSc patients.
Currently there are very limited treatment options for SSc-ILD.
Our lab aims to identify the mechanisms that lead to lung remodeling in SSc-ILD in order to develop new therapies to interrupt the progression of disease and reduce mortality. Our lab also seeks to identify major molecular drivers of skin fibrosis in SSc.

Pulmonary Hypertension (PH)
PH is a common complication of chronic lung diseases including IPF.
PH can also be associated with cardiovascular conditions such as left-heart disease or appear completely own its own.
In all of these cases the diagnosis of PH is strongly associated with increased mortality and there are limited treatment options.
The mission of our lab is to understand the mechanisms that lead to the development of PH in order to discover new treatments
Why Join The Lab?
Chronic Lung Diseases represent the third leading cause of death in the USA.
remained unaffected over the last decade.
Skin and lung fibrosis in SSc patients represent primary sources of morbidity and mortality in SSc. However, treatment options for these patients remain limited.
Our Research
What are the mechanisms that lead to chronic lung and skin disease?
Our research efforts focus on four main topics:

1. Sine Oculis Homeobox Homology 1 (SIX1) in fibrotic diseases
Fibrotic disorders such as IPF and SSc, diseases of particular interest to our lab, display abnormal expression of developmental genes which have been shown to contribute to the fibrotic response.
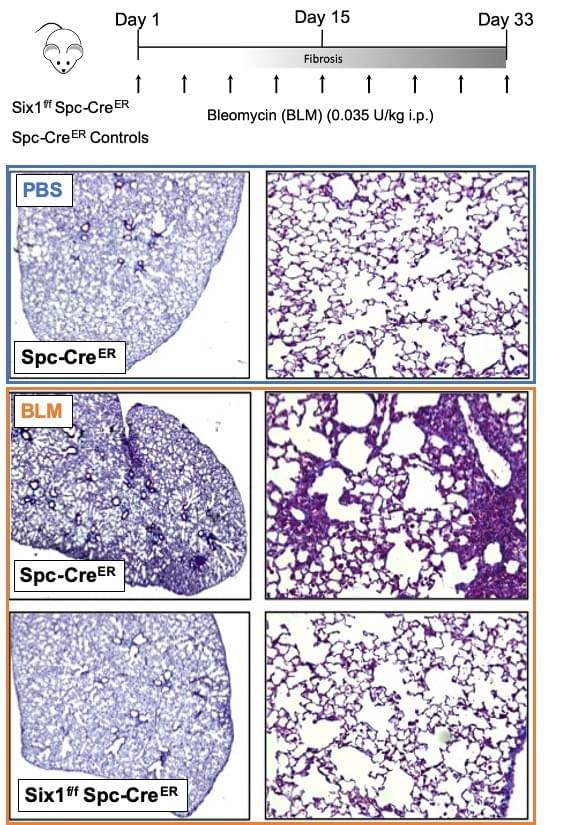
Sine Oculis Homeobox Homolog 1 (SIX1) is a transcription factor normally expressed during embryogenesis where it expands progenitor cell populations and inhibits differentiation. However, in healthy adults, SIX1 is negligibly expressed. We have found that SIX1 is aberrantly expressed in IPF lungs and SSc skin. In IPF lungs, SIX1 localizes to alveolar type II epithelial cells (AT2 cells), identified by co-localization with the AT2 cell-specific expression of surfactant protein C (SPC). When challenged with bleomycin, mice lacking expression of SIX1 in SPC-expressing AT2 cells have reduced lung fibrosis. These protective effects are observed before and after induction of bleomycin-induced lung fibrosis, supporting the therapeutic potential of SIX1 inhibition in IPF. Further, SIX1 is also over-expressed in the fibrotic skin of SSc patients as well as in the subcutaneous bleomycin model of skin fibrosis. Our lab is currently investigating the protective effects of SIX1 knock-out in skin fibrosis.

2. Understanding how changes in the lung extracellular matrix (ECM) lead to vascular remodeling in PH associated with IPF
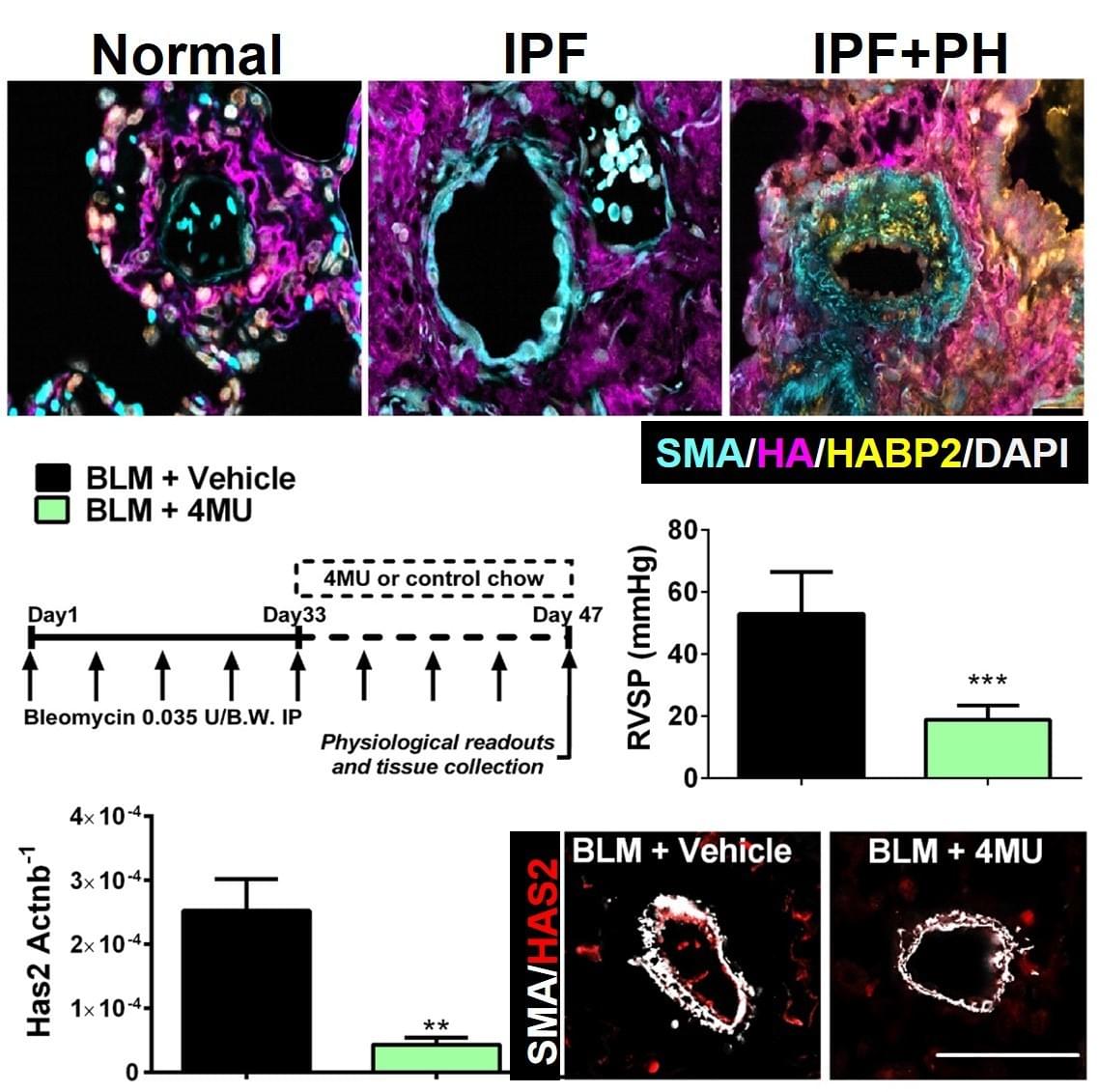
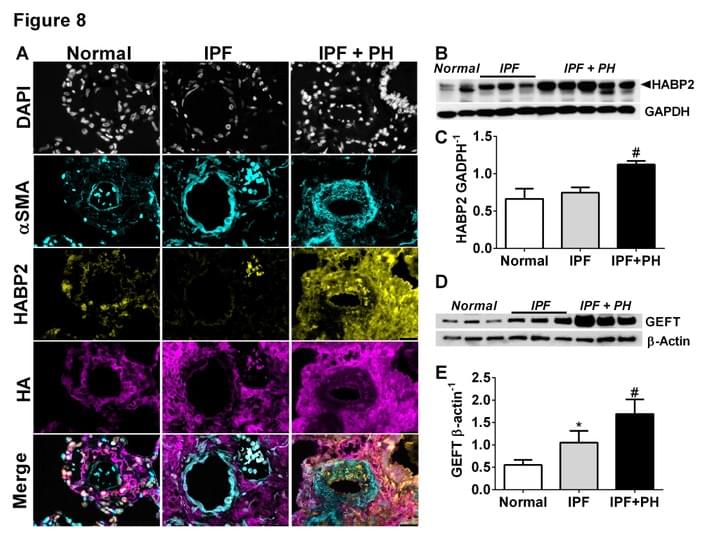
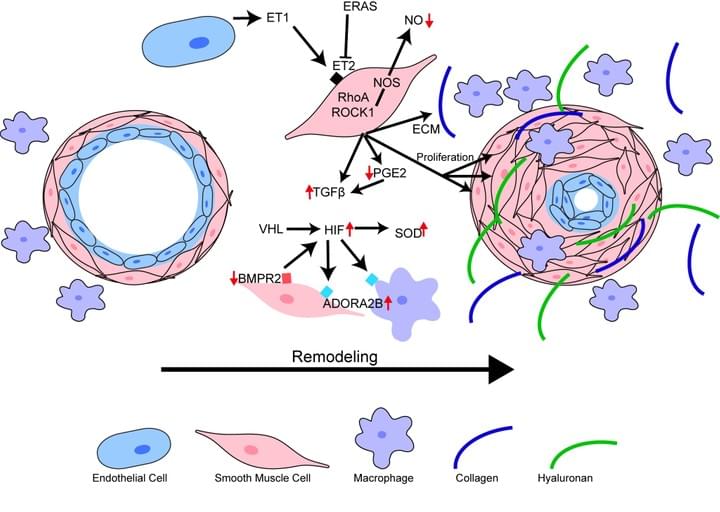
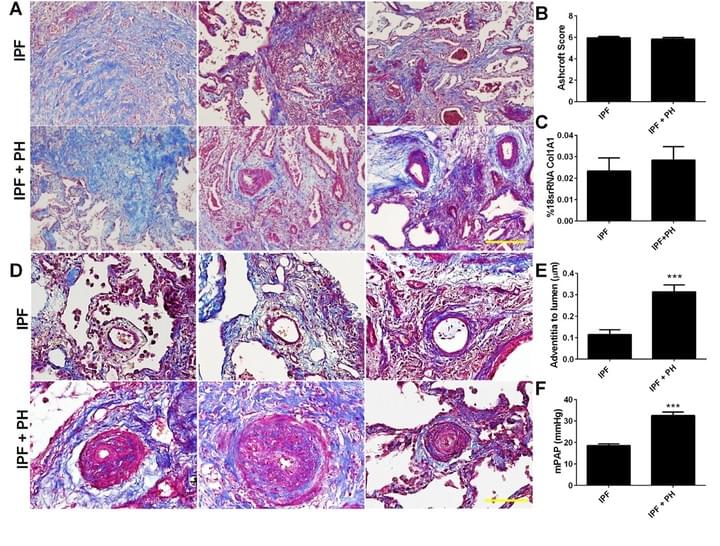
The presence of PH in patients with IPF is the single most significant predictor of mortality, yet there are no cures at present that effectively prevent or treat PH in patients with IPF. In this project we have identified increased levels of hyaluronan, a component of the lung ECM, in the lung of patients with a diagnosis of PH associated with IPF. We next demonstrate that the increase in hyaluronan is mediated by an elevation in hyaluronan synthase 2 (HAS2), an enzyme that makes hyaluronan. Finally we demonstrate that treatment with 4 methyl-umbelliferone (4MU), a drug that inhibits HAS2, is able to reduce levels of hyaluronan and treats PH associated with lung fibrosis. We are currently examining the mechanisms that lead to HAS2 increases in patients with IPF and examining how selective deletion of HAS2 from smooth muscle cells contributes to the development of PH.

3. Determining the contribution of adenosine signaling in skin fibrosis
Skin fibrosis is the most common manifestation of systemic sclerosis (SSc) and is responsible, in large part, for the considerable morbidity of this disease. However there are no FDA-approved treatments for SSc skin fibrosis.
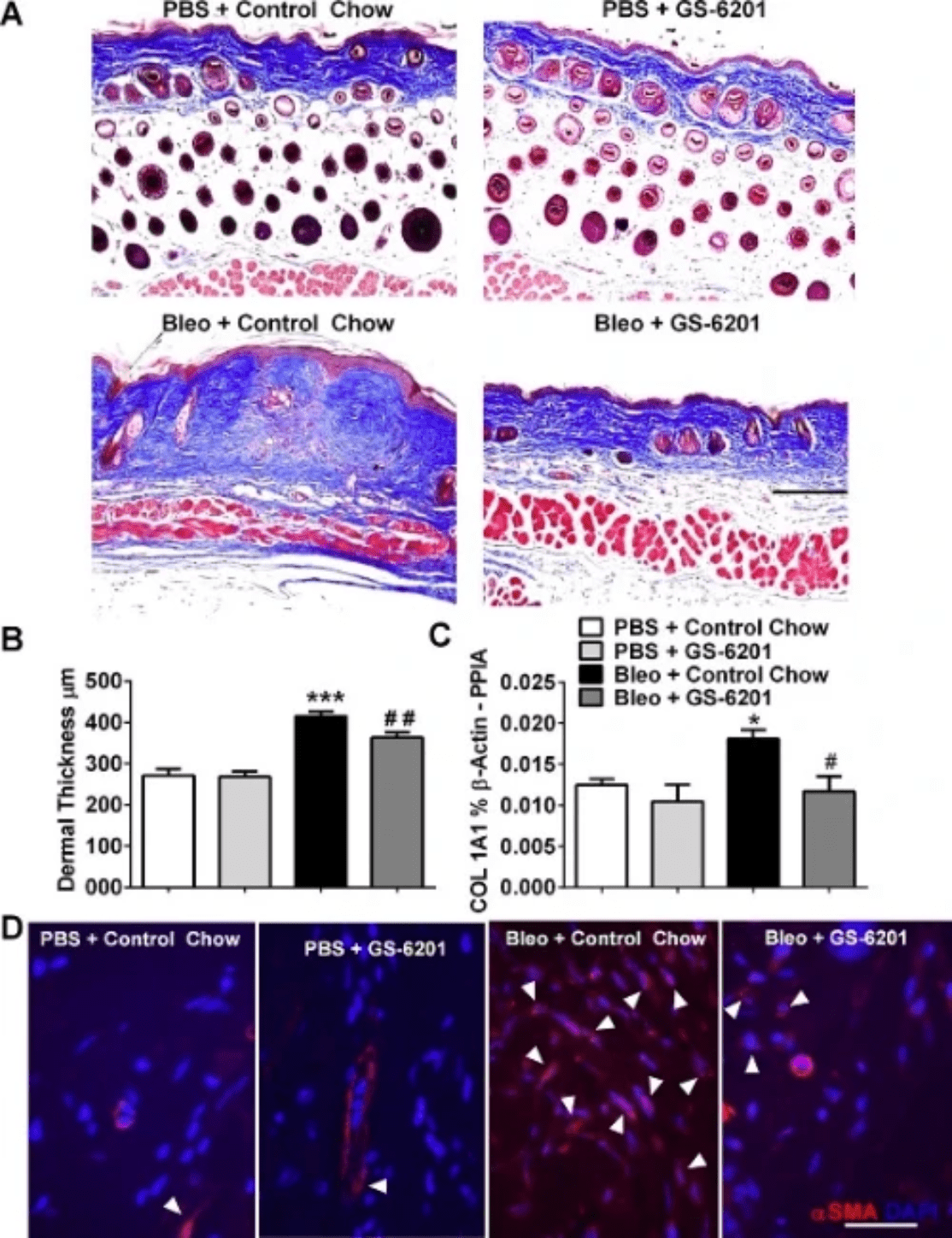
We have demonstrated the key role of adenosine signaling via the ADORA2B receptor in skin fibrosis. Firstly, treatment with the ADORA2B antagonist GS-6201 attenuated dermal fibrosis and the number of alternatively activated macrophages upon exposure to the pro-fibrotic agent bleomycin. Adenosine signaling increases production of the extracellular matrix component hyaluronan. Further, treatment with GS-6201 attenuated hyaluronan production and levels of the hyaluronan-producing enzyme hyaluronan synthase 2 (HAS-2) in mice exposed to bleomycin. Lastly, SSc myofibroblasts overexpress HAS-2 and ADORA2B and increased hyaluronan deposition is observed in SSc skin. Our findings represent key supporting evidence for the investigation of adenosine signaling antagonists as novel therapies for SSc skin fibrosis.

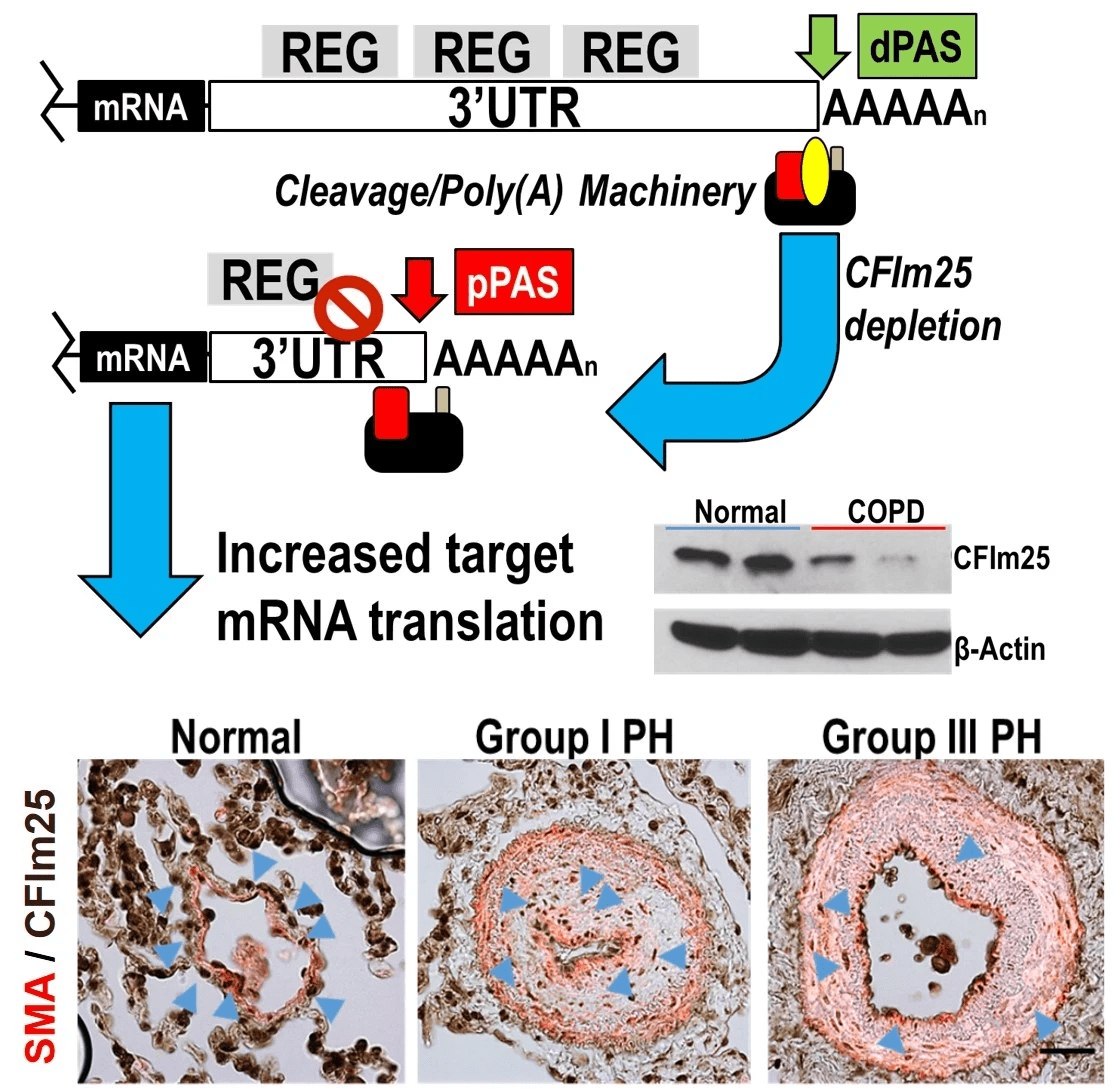
4. Evaluating how changes in mRNA 3’UTR length participates in the development of pulmonary hypertension.
Pulmonary hypertension is a disorder of the pulmonary vasculature defined by increased mean pulmonary arterial pressure (mPAP) leading to right-sided heart failure and ultimately death within 3 to 5 years after diagnosis on average. It can appear on its own or in association with chronic lung diseases. This project examines the mechanisms by which a process known as alternative polyadenylation leads to 3' UTR shortening contributing to vascular remodeling in pulmonary hypertension.
Publications
Our most recent and selected publications are highlighted here
Adenosine and Hyaluronan modulate lung fibrosis and pulmonary hypertension in Combined Pulmonary Fibrosis and Emphysema (CPFE)
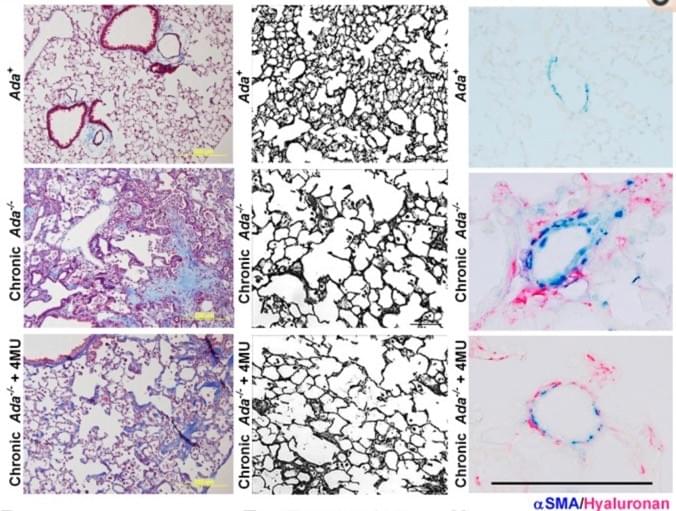
Representative Masson's Trichrome images showing fibrotic deposition in Ada−/− mice compared to Ada+ mice (left). Images of the lung parenchyma from Ada+ mice, Ada−/− and Ada−/−+4MU-treated mice (middle). Representative dual-IHC staining for αSMA and hyaluronan showcasing arterioles from Ada+ , Ada−/− and Ada−/−+4MU-treated mice.
We have generated pre-clinical data demonstrating the capacity of 4MU, a FDA-approved drug, to attenuate features of CPFE in an experimental model of chronic lung injury.
Alterations in cardiovascular function in an experimental model of lung fibrosis and pulmonary hypertension
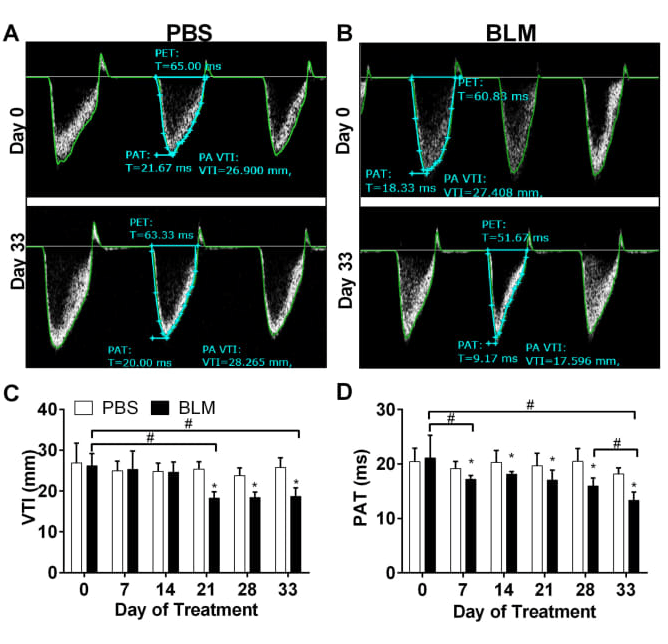
Pulmonary Artery Dynamics derived from Pulsed wave Doppler echocardiography showing velocity time integral (VTI) and pulmonary acceleration time (PAT).
We demonstrate that bleomycin (BLM) treatment leads to a decline in PAT as early as day 7 and 14 after BLM treatment that reduces further by day 33. VTI levels decline
starting on day 21.
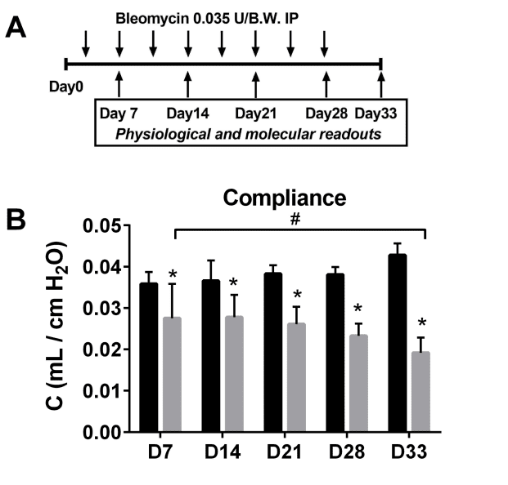
Low-dose administration of bleomycin leads to early alterations in lung mechanics.
Experimental Design and longitudinal compliance values
Here we demonstrate that chronic low-dose bleomycin exposure leads to changes in lung function, illustrated by compliance (C), as early as in day 7 that worsen by day 33. Lung function measurements were performed longitudinally using the Flexivent to identify changes in lung mechanics.
Switching-Off Adora2b in Vascular Smooth Muscle Cells Halts the Development of Pulmonary Hypertension
Vascular ADORA2B expression modulates PH
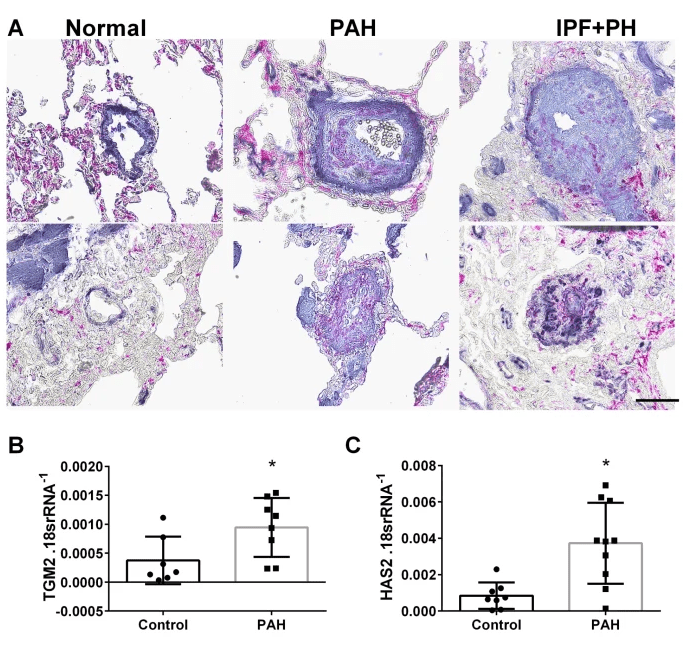
Using mice lacking ADORA2B in smooth muscle cells, we demonstrate that this receptor is necessary for the development of PH induced by bleomycin or by hypoxia-sugen. The novel mechanism involves Adora2b-mediated increased expression of tissue trans glutaminase (TGM2) and hyaluronan synthase 2 (HAS2).
Inhibition of Hyaluronan Synthesis Attenuates Pulmonary Hypertension Associated with Lung Fibrosis
Elevated vascular hyaluronan leads to pulmonary hypertension in the context of lung fibrosis
Here we identify a unique mechanism leading to pulmonary hypertension in the context of lung fibrosis that is mediated by elevated vascular hyaluronan. Here we also show how the hyaluronan synthesis inhibitor, 4MU, prevents the development of pulmonary hypertension.
Pulmonary Hypertension Associated with Idiopathic Pulmonary Fibrosis: Current and Future Perspectives
Here we review the current understanding of the mechanisms known to lead to Group III pulmonary hypertension
In this review we have chosen to focus on the current understating of PH in IPF, we will revisit the main mediators that have been shown to play a role in the development of the disease. We will also discuss the experimental models available to study PH associated with lung fibrosis and address the role of the right ventricle in IPF.
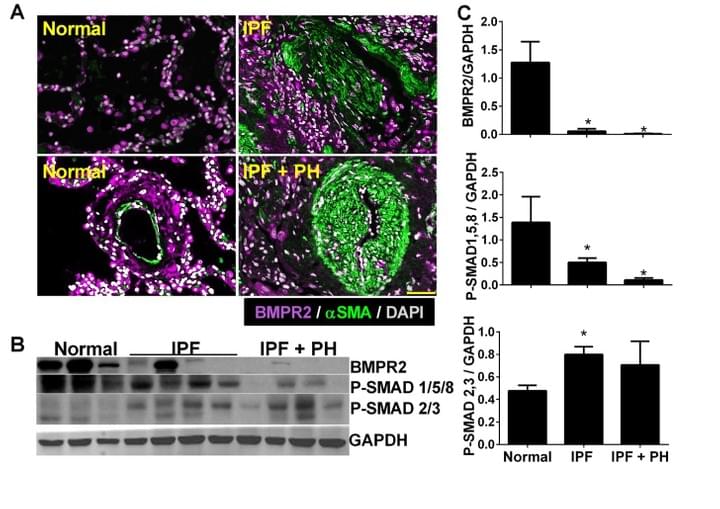
Macrophage bone morphogenetic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension
This is one of the first papers to identify depletion of BMPR2 in IPF
Here we demonstrate that BMPR2 is depleted in IPF and in experimental lung fibrosis. We also demonstrate that IL-6 signalling leads to a cascade of micro-RNAs (miRs) that target the BMPR2 mRNA for degradation.
Altered Hypoxic-Adenosine Axis and Metabolism in Group III Pulmonary Hypertension.
Elevations in succinate activate the hypoxic-adenosine axis
Here we show that alterations in metabolism in patients with IPF and PH lead to heightened stabilization of hypoxia-inducible factor 1 (HIF-1A). HIF-1A then drives the expression of CD73 resulting in augmented adenosine production and signaling through the adenosine A2B receptor (ADORA2B), modulating disease progression.
Click on the link to access all of our published work
on Pubmed
Our group has published over 40 articles in
leading scientific journals.
Team Members
Get to know us!
Harry Karmouty-Quintana, PhD
Harry received his undergraduate degree in Pharmacology
from King’s College London (UK) in 2003. In 2006, he received his PhD in Pharmacology and Biophysics from King’s College London (UK) where he pioneered the use of Magnetic Resonance Imaging (MRI) as a tool to non-invasively image experimental lung disease. His PhD was a joint venture between King’s College London and Novartis Pharmaceuticals, located in Basel, Switzerland. From 2006-2010 he was a Postdoctoral Fellow at McGill University where he was funded by the Fonds de la Recherche en Santé du Québec (FRSQ). He then joined UTHealth in 2010 as Postdoctoral Fellow where he was funded by the American Heart Association (AHA). He was promoted as a Research Track Assistant Professor in 2012 and in 2015 he became a Tenure-Track Assistant Professor at UTHealth. Originally from Barcelona, he may be hard to find if FC Barcelona is playing a Tournament Final!

Weizhen Bi, MD
Senior Research Associate
Weizhen is originally from Liaoning Province in China. She joined the Karmouty Lab in September 2017. Weizhen completed her MSc and MD at China Medical School in ShenYang. She joined The McGovern Medical School at UTHealth as a postdoctoral fellow in 1993 and has since gained extensive expertise in tumor cell biology, vascular development and atherosclerosis. Since 2001, she worked in Dr. Joseph Alcorn's lab at UTHealth where she gained experience in lung cell biology and the role of surfactants in lung immunology. On her free time Weizhen enjoys spending time with her family and preparing traditional Chinese dishes.

Scott D Collum
Lab Manager
Scott is from Huckaybay, TX where he graduated first in his class from Huckaybay ISD and where he learned to raise farm animals. He then moved to college station where he graduated summa cum laude with a B.S. in Biochemistry from Texas A&M University. He then moved Houston to pursue his PhD at UTHealth in RNA biology and pulmonary diseases. Scott is an avid runner and completed his first marathon in December 2016. If you are looking for the best donuts in town, he is the person to ask!

Sarah Shin
MD/PhD Student
Sarah is from St. Louis, MO. She graduated summa cum laude from the University of Missouri-Kansas City with a BS in Biology and minors in Chemistry and Math. She joined the UTHealth/MD Anderson/UPR Medical Scientist Training Program in 2019. She intends to specialize as an intensivist-anesthesiologist. Her research interest is lung alveolar epithelial cell behavior in pulmonary fibrosis-like hydrogels. Her hobbies include exploring coffeeshops, learning new sports, and watching reality TV.

René Girard
PhD Student
Rene is from Corquin Honduras and grew up in New Orleans, LA. He graduated from LSU with a bachelor's of science in Biology. He then completed a Master's at the University of Texas Health Science Center at Tyler on a project relating Depression to Inflammation. His current research looks at the effect that lung diseases play on alternative polyadenylation. His hobbies are Magic the Gathering, hangin out with his cats, and making fun of Sarah.

Janani Subramaniam
Postdoctoral Fellow
Jan earned her Bachelors degree in Biology & Psychologyfrom Roger Williams University in Bristol, Rhode Island. She then made her way
to Texas and got her Masters and PhD from MD Anderson UTHealth Graduate School of Biomedical Sciences, studying ankyrin proteins in cardiac biology. Currently, she is studying Alternative Polyadenylation (APA) in Pulmonary Hypertension (PH) and associated Right Ventricular (RV) failure. Jan is passionate about making science accessible to general audiences, and improving patient health
outcomes by driving scientific strategy and clinical development. Outside of work, she enjoys spending time with her friends, family, and her puppy, Clicquot. And she has never been known to say no to crème brulée or champagne.
Jamie Tran
PhD Student
Jamie is from Los Angeles,CA and grew up in Houston, TX. They graduated from the University of Houston with a Bachelor of Arts in Psychology with a minor in Sociology. After that, they then completed a Master’s at the University of Texas Health Science Center in Houston on a project focusing on circadian rhythm restoration in cancer cells and behavior. Currently, their research interests are examining systemic changes in NUDT21 and hyaluronan in neurodegenerative states such as Alzheimer’s and Alcohol Use Disorder. In their spare time they enjoy dog training for lunch money, wandering around farmer’s markets, and singing in the car.

Phoebe Andrew
Visiting Master's Student
Phoebe Andrew is a visiting student from the UK. She studies pharmacology at King's College London. Her project focuses on using spatial transcriptomics to study lung disease. She loves sewing and baking in her spare time.
Previous Team Members
We are very grateful for the contributions of our previous lab members!

Tinne C Mertens, PhD
Scientist at Galapagos NV
After she finished her Master degree in biomedical sciences at Hasselt University in Belgium, Tinne moved to Amsterdam in the Netherlands and started her PhD training at the Leiden University Medical Center. During her Master and PhD training, she worked extensively with in vitro air-liquid interface airway epithelial cell cultures of both animal and human origin. Next, she moved to Houston and joined the lab in June 2016 where she had the opportunity to work with in vitro cultures of human transplant and explant tissues in addition to translational experimental models of chronic lung disease. Combining in vitro cultures and in vivo models allowed her to investigate of role specific cell types in the development of chronic lung disease and identify possible therapeutic targets. Tinne now works as an in vitro scientist at Galapagos NV!

Wang Wei, MD
Cardiothoracic Surgeon in Wuhan, China
Wang Wei is a cardiothoracic surgeon from the Renmin Hospital of Wuhan University, China. While a member of our lab, he worked to uncover new strategies to treat acute respiratory distress syndrome (ARDS) and identify new treatments for cor pulmonale in chronic lung diseases. When the COVID-19 epidemic struck his community of Wuhan, Wang Wei returned to China to serve his community as a physician.

Cory Wilson
Internal Medicine Resident
Cory is from Little Elm, TX. He completed his BSc in Biochemistry from the University of Houston. He then joined the MD/PhD program at the MDAnderson / UTHealth Graduate School of Biomedical Sciences, he joined the Karmouty Lab in September 2017 where his project aimed to identify developmental re-programming in chronic lung disease. Cory enjoys cooking and is a BBQ connoisseur.

Ankit Hanmandlu
Internal Medicine Resident
Ankit is a former medical student at McGovern Medical School. He is a graduate of the University of Texas at Austin. He currently works on investigating pathogenic mechanisms of pulmonary hypertension and pulmonary fibrosis. He is an internal medicine resident in Detroit Med Center MI.

Lucy Revercomb
Undergraduate Student
Lucy is from Martinsville, NJ. She is currently an undergraduate student at Rice University working towards a BS in biochemistry with a certificate in Spanish. She joined the Karmouty Lab in August 2019, where her research focuses on the pathogenesis of pulmonary fibrosis and pulmonary hypertension. Outside the lab she enjoys playing tennis, gardening, and painting!

Nancy Wareing
Internal Medicine Resident at Emory
Nancy is from Austin, TX. She received her BS in Biology from Texas Christian University. She joined the UTHealth/MDAnderson/UPR Medical Scientist Training Program in 2015. She intends to specialize in Rheumatology. Her research interest is the role of aberrant expression of developmental genes in skin fibrosis. Her hobbies outside the lab include painting, martial arts, and cooking! She graduated from the MD/PhD program in 2023.

Andrew Peters
Dr. Andrew Peters studied biomedical engineering at Texas A&M University focused on vascular fluid mechanics and pulmonary vascular disease. After a more technical internship in mechanical engineering at the University of Duisburg-Essen, he matriculated into the MD/PhD program at UT Houston. He looked into nanotechnology before moving into medical genetics focusing on cell signaling and aortic disease. He gained clinical experience in vascular surgery at WHC/GUH residency program before moving back to research where he continues to follow his pulmonary and critical care interests.
News
Recent accomplishments from the Lab

New Foundation Funding!
PI: Nancy Wareing
The Role of SIX1 in Skin Fibrosis in Systemic Sclerosis
Nancy was awarded the Rheumatology Research Foundation Future Physician Scientist Award!
The most common clinical feature of systemic sclerosis (SSc; scleroderma) is skin thickening and tightening, caused by deposition of excess extracellular matrix material in the skin. There are no FDA-approved drugs for skin involvement in SSc, primarily due to the fragmented understanding of its driving pathophysiologic mechanisms. This underscores the urgent need to identify novel targeted therapies for the treatment of SSc. Nancy identified the upregulation of a novel developmental transition factor, Sine Oculis Homeobox Homolog 1 (SIX1) in the skin of persons with SSc but not age- gender- race-matched healthy persons, and localized this protein to keratinocytes, the primary epithelial cell in the epidermis. SIX1 has been shown to drive epithelial to mesenchymal transition (EMT) in development and numerous disease states. I demonstrated that SIX1 correlates with markers of EMT in SSc skin biopsies, strongly supporting a driving role of SIX1 in skin fibrosis by promoting EMT. This project seeks to identify a novel pathogenic role of an unknown developmental transcription factor, SIX1 in the progression of skin fibrosis and represent a potential new therapeutic target for this potentially devastating disease.

New NIH fellowship funding!
PI: Cory Wilson
Pulmonary Fibrosis: Novel Role for Developmental Gene Six1 in Alveolar Type II Cells
Cory Wilson was awarded a F30 NIH fellowship grant from National Heart, Lung, and Blood Institute!
Idiopathic pulmonary fibrosis (IPF) is the most common idiopathic interstitial pneumonia with a median survival time of 2-4 years after diagnosis. The alarming mortality rate is due to the lack of effective treatments. IPF is a chronic, fibrotic disease that is characterized by alveolar destruction due to increasing extracellular matrix deposition that leads to poor lung compliance, impaired gas exchange, and ultimately respiratory failure. In this proposal we aim for the first time to identify the mechanisms whereby Six1 modulates fibrosis.Thus, we propose to elaborate and evaluate these novel findings. We propose to identify the molecular mechanisms by which Six1 acts on AECII cells and how this contributes to the pathophysiology of pulmonary fibrosis with the hypothesis that increased Six1 expression in AECII cells contributes to the development of pulmonary fibrosis.

1st Place ACP National Abstract Competition
Congratulations Cory!
ACP Internal Meeting Philadelphia April 11-13, 2019

New DoD CDMRP Funding!
PR181329
Disrupting Six/Eya signalling as new therapy for lung fibrosis
Lung diseases are currently the third killer in the US, behind cancer and cardiovascular conditions, yet there is a lack of effective therapies for lung diseases. Amongst lung diseases, lung fibrosis is a common condition. A defining feature of lung fibrosis is shortness of breath that is a result of the lung being unable to oxygenate blood effectively. This happens as a result of scarring in the alveolar spaces, which obliterates these airspaces where oxygen is able to diffuse into the red blood cells and oxygenate the body. Although we now understand the environmental, workplace and genetic predispositions that may lead to lung fibrosis, many of the mechanisms that promote and maintain this aberrant repair process are not known. There are very limited treatment options for patients with lung fibrosis, which accounts for approximately 50% of all lung transplantations. This highlights the need to develop new treatments for lung fibrosis.
Novel findings have identified a molecule known as Sineoculis homeobox homolog 1 or Six1 to be elevated in patients with lung fibrosis. Six1 is only normally observed in the embryo where it plays a role in the development of the lungs, therefore its presence in adult lungs with lung fibrosis is abnormal. Remarkably, Six1 was observed to be present in lung epithelial cells from patients with IPF.

New AHA Funding!
18IPA34170220
Ex-Vivo Lung Perfusion in Acute Lung Injury
Pulmonary diseases are the 3rd largest killer in the US and include acute respiratory distress syndrome (ARDS), a fatal disease characterized by widespread inflammation and loss of barrier function of the lungs resulting in accumulation of fluid that leads to loss of gas exchange and subsequent death. Despite ongoing research efforts there are no pharmacological agents to treat ARDS. Specifically the adenosine signaling pathway plays a critical role however systemic treatments have significant side effects. Subsequently, treatment for ARDS is only supportive with mechanical ventilation, fluid management and extracorporeal membrane oxygenation (ECMO) for severe ARDS. Yet, novel primary care strategies are lacking so mortality is high with 40-45% of ARDS patients dying.
It is essential for us to develop novel therapies for the treatment of ARDS in order to reduce the mortality of this fatal disease.
Collaborations
At the forefront of Pulmonary Disease Research through access to highly valuable patient material and solid collaborations with physician scientists.

Clinical Collaborations
Memorial Hermann and Houston Methodist Research Institute
We collaborate closely with physician scientists at Memorial Hermann Hospital and Houston Methodist Hospital systems, making advances in translational research. Through these collaborations, Dr. Karmouty Quintana directs the UTHealth Pulmonary Center of Excellence lung tissue biobank. This Biobank is one of the largest in the country and it houses lungs from patients with COPD, IPF, Pulmonary Hypertension in addition to other lung diseases.

Scientific Collaborations
Local and International Network of Collaborators
We collaborate with many basic-science laboratories locally and internationally to advance our understanding of how lung diseases develop with the hope of developing new treatments for disease. Current collaborations include the use of nanoparticles for gene-therapy for lung injury, microbiome research in patients with chronic lung injury, applied bio-informatics for lung research, and analysis of systemic sclerosis patient cohorts.
Opportunities
Do you want to be part of our Research Team? Contact us!
We're interested in hearing about talented scientists!
Funding
Current and Past Funding

NIH
1R01 HL138510-01 (PI: Karmouty-Quintana)
3’UTR shortening in Pulmonary Vascular Disease
The goal of this project is to examine the effects of alternative polyadenylation in the development of pulmonary hypertension.

AHA
24TPA1303921 (PI: Karmouty-Quintana)
Linking NUDT21 Depletion to 3'UTR Shortening andCerebrovascular Dysfunction in Alzheimer's Disease

NIH
R01HL157100-01 (PI: Karmouty-Quintana)
The Role of Sineoculis Homeobox Homolog 1 (Six 1) in PulmonaryFibrosis

Innovative Project Award
18IPA34170220 (PI: Karmouty-Quintana)
Ex-Vivo Lung Perfusion in Acute Lung Injury
The project focused on evaluating the capacity of EVLP approaches as a novel approach to treat ARDS.

Biomedical Research Grant
RG-414673 (PI: Karmouty-Quintana)
Alternative Polyadenylation in the pathogenesis of COPD
The goal of this project is to examine the contribution of alternative polyadenylation in the pathogenesis of COPD

Call for Grants Notification in IPF
G-45857 (PI: Karmouty-Quintana)
BMPR2 in Acute and Chronic Lung Injury.
The goal of this project is to examine the contribution of bone morphogenic protein receptor 2 (BMPR2) in acute and chronic lung injury

Scientist Development Grant
14SDG18550039 (PI: Karmouty-Quintana)
The role of hyaluronan in pulmonary hypertension secondary to lung fibrosis
This project focuses on understanding the contribution of hyaluronan in the development of pulmonary hypertension secondary to chronic lung diseases.

Entelligence MD Actelion Award
2013 (Karmouty-Quintana)
The role of hyaluronan in pulmonary hypertension associated with idiopathic pulmonary fibrosis (IPF)
The project focused on understanding the contribution of hyaluronan in the development of pulmonary hypertension secondary to chronic lung diseases.
© 2020